Capacity fading of lithium battery cathode materials is a common problem during the use of lithium batteries. It will cause the battery capacity to gradually decrease, thus affecting the service life and performance of the battery. The reasons for capacity fading are very complex and involve many aspects such as structural changes in materials, loss of electrolyte, and reactions at the electrode interface. Here we discuss the mechanism and influencing factors of capacity fading of lithium battery cathode materials, and introduce some methods to delay capacity fading.
1. Factors causing capacity attenuation of lithium battery cathode materials
(1) An important reason for capacity fading is the structural change of the cathode material
During the intercalation and deintercalation of lithium ions, the volume of the cathode material changes, which can lead to structural changes such as microcracks, powder aggregation, and particle separation in the material. These structural changes reduce the material’s conductivity and ion diffusion rate, reducing the available active material surface area, thereby reducing the battery’s capacity. In addition, structural changes in the cathode material will also lead to stress changes inside the battery, further exacerbating the degree of capacity fading.
(2) Solvent loss in the electrolyte also causes capacity fading
During the charge and discharge cycle, the solvent in the electrolyte will gradually decompose and oxidize, forming a solid electrolyte interface layer (SEI layer). The formation of this interface layer is inevitable during normal battery operation, but too much interface layer will hinder the transport of lithium ions and electrode reactions, resulting in a decrease in battery capacity. In addition, the loss of solvent in the electrolyte will also cause changes in the electrolyte concentration inside the battery, affecting the performance and stability of the battery.
(3) The reaction at the interface between the electrode and the electrolyte will also cause capacity fading.
The electrolyte salt on the electrode surface will react with the solvent in the electrolyte to form a layer of electrolyte salt deposits, which is called the “dead zone” of the electrolyte salt. This layer of dead space will reduce the available area of active material on the electrode surface and limit the capacity of the electrode. In addition, the reaction at the interface between the electrode and the electrolyte will also trigger a series of side reactions, such as redox reactions, solvent dissociation, and deintercalation reactions of electrode materials, which further aggravates the degree of capacity fading.
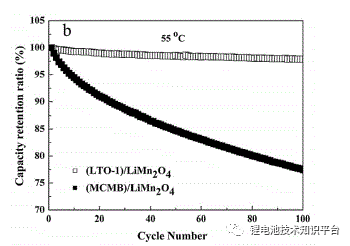
2. Methods to delay capacity fading
In order to delay the capacity decay of lithium battery cathode materials, some measures can be taken.
(1) Optimize the structure and composition of cathode materials
By changing the crystal structure, particle size and morphology of the material, the volume change of the material can be reduced and the impact of structural changes on battery performance can be reduced. In addition, suitable additives and dopants can improve the conductivity and ion diffusion rate of the material, and increase the capacity and cycle stability of the battery.
(2) Optimize the electrolyte formula and additives
Appropriate selection of solvents and salts can reduce the decomposition and oxidation rate of the solvent in the electrolyte and reduce the loss of the electrolyte. In addition, adding an appropriate amount of additives can improve the stability of the electrolyte and the formation of the interface layer, and reduce the capacity fading of the battery.
(3) Reasonable charge and discharge control and temperature management can also delay the rate of capacity fading.
Controlling the charge and discharge current and voltage of the battery to avoid too high or too low charge and discharge rates can reduce the reaction at the interface between the electrode and the electrolyte and the structural change of the electrode material. At the same time, properly controlling the operating temperature of the battery and avoiding excessively high or low temperatures can slow down the decomposition of the electrolyte and the loss of the solvent, and improve the cycle stability of the battery.
Capacity fading of lithium battery cathode materials is an inevitable problem during the use of lithium batteries. The mechanism of capacity fading is very complex and involves many aspects such as structural changes in materials, loss of electrolyte, and reactions at the electrode interface. In order to delay the rate of capacity fading, the performance and cycle stability of the battery can be improved by optimizing the structure and composition of the cathode material, optimizing the formula and additives of the electrolyte, and rationally controlling charge and discharge conditions and temperature management. With the continuous advancement of science and technology, I believe that research and solutions to the problem of lithium battery capacity fading will achieve better results.