With the rapid development of science and technology, lithium batteries have become one of the important energy sources for modern electronic equipment. During the use of lithium batteries, lithium batteries may face the problem of lithium precipitation, which not only affects the performance of the battery, but may also pose a threat to the safety of the battery. This article will deeply explore the causes, effects and countermeasures of lithium precipitation in lithium batteries.
1. Concepts and definitions
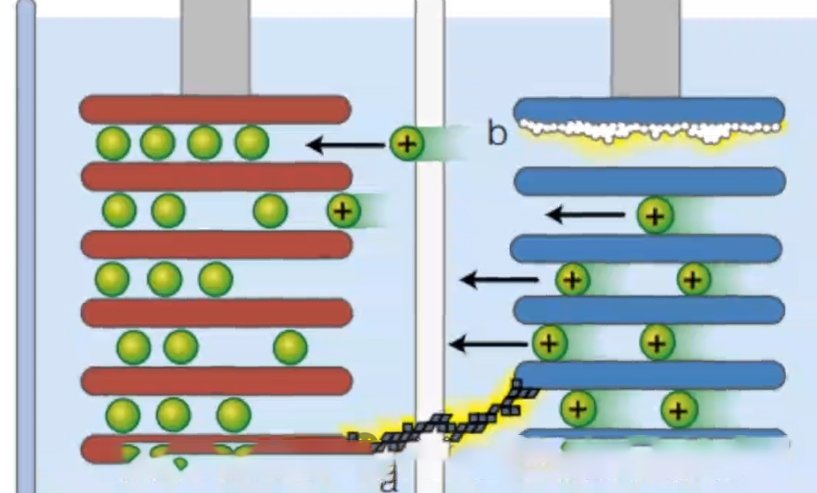
Lithium precipitation in lithium batteries refers to the phenomenon that lithium metal in lithium-ion batteries precipitates on the surface of the negative electrode under certain conditions. Usually in low temperature environments, or when the battery is repeatedly charged or subjected to external forces, lithium ions will undergo an irreversible reaction to form solid metallic lithium. The precipitated metallic lithium often exists in the form of dendritic crystals, which are called lithium dendrites.
2. Reasons for lithium precipitation in lithium batteries

Lithium precipitation in lithium batteries mainly occurs during the charging process. When the battery is charged, lithium ions in the positive electrode material will migrate to the negative electrode through the electrolyte and deposit on the negative electrode to form lithium metal. During this process, if the charging conditions are improper, such as too high charging voltage or too low electrolyte concentration, it may cause excessive deposition of lithium metal on the negative electrode and form lithium dendrites. The growth of lithium dendrites will destroy the solid electrolyte interface (SEI) film inside the battery, resulting in reduced battery performance and possible safety issues.
3. The impact of lithium precipitation in lithium batteries
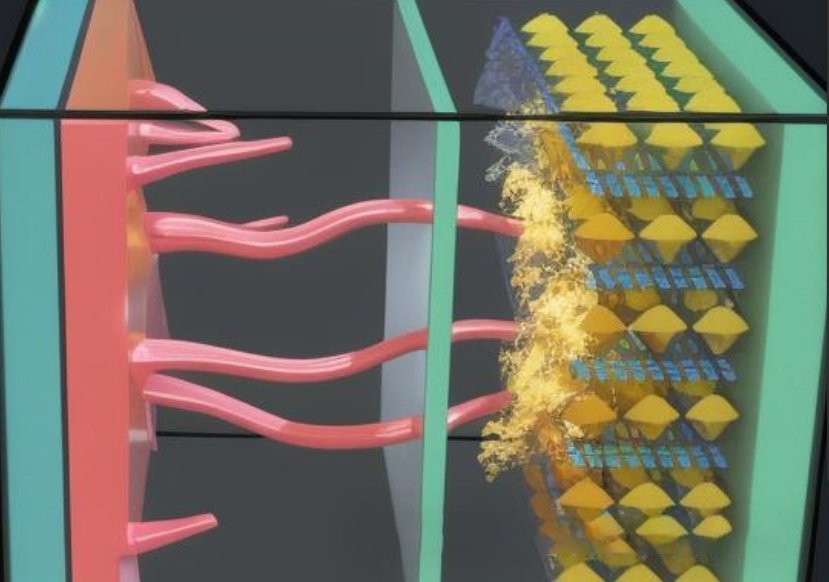
1. Reduced battery performance: The growth of lithium dendrites will increase the internal resistance of the battery and reduce the energy density and charge and discharge efficiency of the battery. In addition, dendrites may puncture the separator and bring the positive and negative electrodes into direct contact, causing the battery to short-circuit.
2. Potential safety hazards: The growth of lithium dendrites will not only destroy the SEI film, but may also puncture the battery separator, causing an internal short circuit in the battery. Short circuits can generate large amounts of heat and current, which may cause safety issues such as fire or explosion.
4. Strategies to deal with lithium precipitation in lithium batteries
1. Optimize charging conditions: By controlling charging voltage and current to avoid overcharge and over-discharge, the risk of lithium precipitation in lithium batteries can be effectively reduced. In addition, the use of smart charging technologies, such as trickle charging and constant voltage charging, can alleviate the lithium deposition problem to a certain extent.
2. Improve electrolyte performance: By adjusting the composition and concentration of the electrolyte, its electrochemical stability and lithium ion transport performance can be improved, thereby reducing the risk of lithium precipitation. In addition, developing new solid-state electrolytes is also an effective solution.
3. Select appropriate cathode materials: The properties of cathode materials have an important impact on the charge and discharge performance and safety of lithium batteries. Choosing cathode materials with high energy density, excellent electrochemical stability and low lithium ion diffusion resistance can reduce the risk of lithium precipitation in lithium batteries.
4. Negative electrode surface coating: Coating the negative electrode surface with a layer of material with good electrochemical stability and lithium ion transport properties can prevent the growth of lithium dendrites and improve the safety of the battery. Commonly used coating materials include carbon black, oxides and polymers.
5. Battery management system: Real-time monitoring and intelligent control of the charging and discharging process of lithium batteries through the battery management system (BMS) can ensure that the battery works under safe conditions and avoid overcharging and over-discharging, thereby reducing analysis. Lithium Risks.
5. Conclusion
To sum up, the problem of lithium precipitation in lithium batteries is a complex and serious problem. To solve this problem, we need to start from many aspects, including optimizing charging conditions, improving electrolyte performance, selecting appropriate cathode materials, negative electrode surface coating, and adopting battery management systems. Only in this way can the risk of lithium precipitation in lithium batteries be effectively reduced and the performance and safety of the battery improved. At the same time, we also need to continue to conduct in-depth research on the mechanism and performance of lithium batteries and develop new, more efficient and safer lithium battery technologies to meet the growing needs and application scenarios. In the future, with the continuous advancement of technology and in-depth research, we have reason to believe that more complete solutions will be proposed and implemented. At the same time, we should also pay attention to following safety regulations when using lithium batteries, correctly using and protecting lithium batteries, and avoiding safety accidents. In this context, we call on scientific researchers and enterprises to strengthen cooperation and work together on the innovation and development of lithium battery technology.